Electroplating: A Comprehensive Guide to Metal Surface Enhancement
When discussing metal surface finishing, anodizing is frequently the first technique that springs to mind, particularly for aluminum. Yet, there exists a more adaptable alternative: electroplating. Unlike anodizing, which is limited to specific metals, electroplating can be applied to a wide array of materials. By depositing a thin metal layer onto a part, it can remarkably enhance the part's appearance, corrosion resistance, durability, and conductivity.
The roots of electroplating trace back to the early 19th century. Italian chemist Luigi Brugnatelli was the first to use electric current to plate gold onto silver. However, it wasn't until the 1830s that British scientists John Wright and George Elkington refined the technique. Their patented method in 1840 marked the beginning of electroplating's widespread industrial adoption. Over the years, the process has expanded to include various metals like copper, nickel, and chromium, enabling manufacturers to safeguard products against corrosion while boosting their visual appeal. Today, electroplating is an indispensable part of modern manufacturing.
This guide delves deep into the intricacies of electroplating, exploring its process, types, benefits, limitations, and the key elements for successful implementation in contemporary industries.
What Is Electroplating?
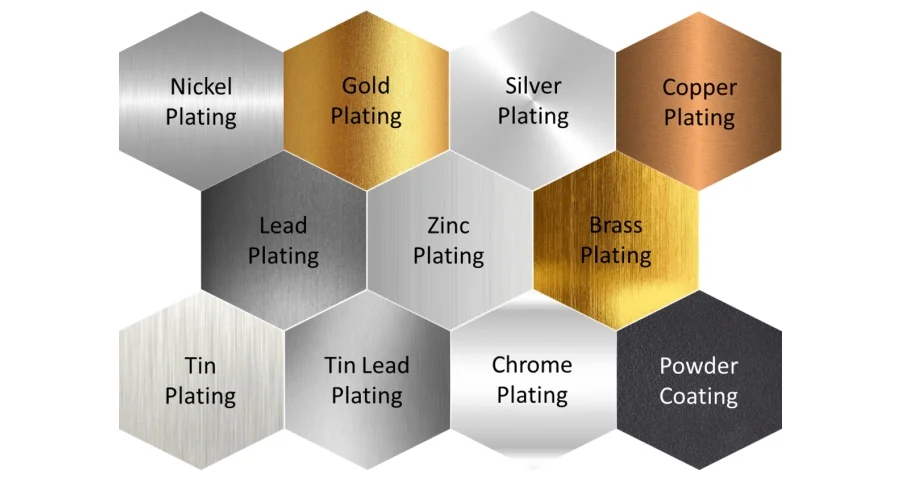
Electroplating is an electrodeposition process that uses an electric current to coat a thin layer of metal (the deposition metal) onto the surface of another material (the substrate). This added layer can enhance the substrate's aesthetic appeal and various properties, including physical attributes like heat and electrical conductivity, mechanical characteristics such as strength and abrasion resistance, and chemical properties like corrosion resistance.
Deposition metals in electroplating are selected based on their unique properties and can be used alone or in combination to achieve desired results on the substrate. Here are some commonly used metals:
Copper: Valued for its conductivity and heat resistance, copper is also frequently used to improve adhesion between material layers.
Gold: This precious metal offers exceptional corrosion, tarnish, and wear resistance. It is highly prized for its conductivity and luxurious appearance.
Silver: With the highest electrical conductivity among all metals, silver also boasts excellent thermal conductivity. It's often used as a more affordable alternative to gold in applications requiring both thermal and electrical conductivity, and its finish adds visual appeal.
Nickel: Nickel provides outstanding wear resistance, which can be further enhanced through heat treatment. It also offers good corrosion resistance, especially when plated onto steel or other substrates. Electroless nickel plating, which uses nickel, results in a low-friction surface with high hardness.
Zinc: Known for its high corrosion resistance, zinc is commonly used to protect steel substrates. When alloyed with nickel, it becomes even more resistant to atmospheric corrosion.
Palladium: A bright metal, palladium is often used as a substitute for gold or platinum due to its excellent corrosion resistance, good electrical conductivity, and cost-effectiveness. Alloying it with nickel improves its hardness and plating quality.
Tin: A matte, bright metal, tin is recognized for its excellent solderability and good corrosion resistance. It's also considered environmentally friendly and generally more cost-effective than many other metals.
Chromium: Chromium provides exceptional hardness and a shiny, mirror-like finish. It also enhances wear resistance and corrosion protection.
It's important to carefully select the substrate and coating, as not all materials are compatible. For example, steel cannot be directly plated with silver; it first needs a layer of copper or nickel before silver can be applied.
How the Electroplating Process Works
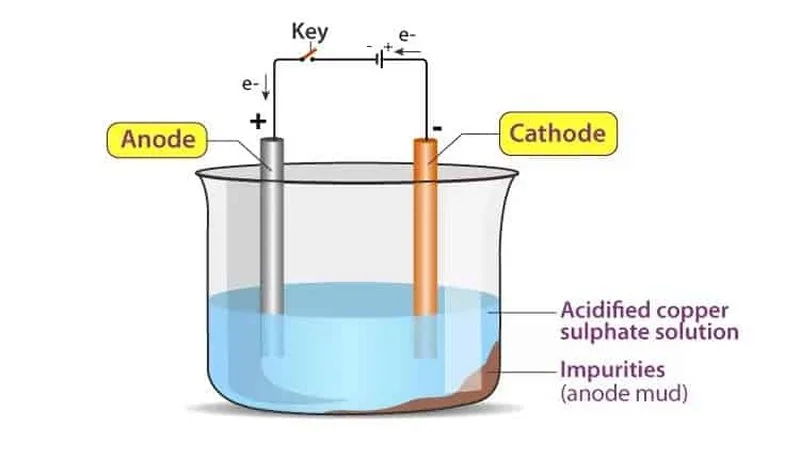
The electroplating process is based on electrochemical principles to deposit a thin metal layer onto a substrate. Let's take copper electroplating as an example to understand the step-by-step process:
Step 1: Preparation
The electroplating setup involves four essential components: the anode, cathode, electrolytic solution, and power source.
Anode: As the positive electrode, it is the metal that will form the plating. It dissolves into the electrolyte, releasing metal ions into the solution.
Cathode: The negative electrode, which is the substrate to be plated. Metal ions from the solution deposit on its surface to form the metal coating. Before electroplating, the substrate must be thoroughly cleaned to remove oils, dust, oxides, and other contaminants. Cleaning methods may include degreasing, acid etching, or ultrasonic cleaning to ensure a smooth, impurity-free surface that allows for uniform metal ion deposition. In some cases, an acidic solution is used to activate the substrate and improve adhesion.
Electrolytic Solution: This is where the electrochemical reaction takes place. It contains one or more metal salts, such as copper sulfate (CuSO₄) or nickel chloride (NiCl₂), serving as both the source of metal ions and the medium for ion conduction.
Power Source: It supplies direct current (DC) for the electroplating process, driving metal ions to deposit on the cathode and form a coating. The current density (the amount of current per unit area) determines the deposition rate and quality, and adjustments to the power source affect the coating's thickness, adhesion, and uniformity.
For example, when plating brass with copper, the brass acts as the substrate and is connected to the negative terminal, making it the cathode. A copper-based solution, like copper sulfate, is used as the electrolyte. When dissolved, this solution releases positive copper ions. A copper anode is used to replenish the copper ions in the electrolyte, ensuring a continuous supply for the plating process.
Step 2: Electrolytic Process
Once the anode and cathode are immersed in the copper sulfate solution and connected to the power source, direct current flows from the power source to the anode. This creates an electric field between the anode and cathode through the electrolyte. The cathode becomes negatively charged (due to an excess of electrons), and the anode becomes positively charged.
In response to the electric field, the positively charged copper ions (Cu²⁺) in the solution are attracted to the negatively charged brass cathode. When they reach the cathode, these ions gain electrons and are reduced to solid copper, which then deposits on the brass surface as a thin copper layer. The reduction reaction at the cathode is: Cu²⁺(aq) + 2e⁻ → Cu(s).
At the same time, the electric current flowing through the anode causes copper atoms to lose electrons (oxidation) and dissolve into the solution as copper ions (Cu²⁺). The oxidation reaction at the anode is: Cu(s) → Cu²⁺(aq) + 2e⁻.
These copper ions (Cu²⁺) move from the anode to the cathode, starting a new cycle of reduction. The electrons lost by copper atoms at the anode travel through the external circuit to the cathode, completing the electrical circuit. As electroplating continues, the copper anode gradually dissolves, continuously replenishing copper ions in the solution and maintaining a stable ion concentration. If a different metal were used as the anode, the copper ions in the solution would not be replenished, leading to a lighter solution color and lower copper sulfate concentration.
Types of Electroplating Methods

There are several electroplating methods, each designed for specific applications and outcomes.
The Benefits of Electroplating
By applying a thin metal layer to a substrate, electroplating significantly improves its physical, mechanical, and chemical properties. Here's how these improvements manifest and their typical industry applications:
Improved Physical Properties (Color, Luster, Conductivity)
Electroplating enhances a substrate's appearance by creating a smoother, shinier surface at a relatively low cost. While metals are naturally conductive, electroplating adds an extra conductive layer, boosting performance without a significant cost increase. It also enables non-metals to be used in electrical applications, reducing both costs and weight, which simplifies transportation and storage.
Consumer Goods Industry: Jewelry and watches often undergo electroplating with precious metals like gold, silver, or rhodium to enhance their luster and market appeal. In household items, chrome or nickel plating makes appliances, cutlery, cookware, faucets, and kettles more attractive and easier to clean.
Defense and Aerospace Industry: Black electroless nickel plating is used to absorb light and reduce surface reflection, which is crucial for manufacturing stealth vehicles and aerospace components that need to evade detection.
Electronics Industry: Gold plating is common in semiconductors, connectors, and switches due to its excellent conductivity and corrosion resistance. Silver, with even better conductivity, is used in wires, contacts, and PCBs for fast signal transmission. Copper, a more affordable option with good conductivity, is widely used in PCBs and electrical connections.
Improved Mechanical Properties (Tensile Strength, Bending Strength, Abrasion Resistance, Surface Finish)
Electroplating strengthens materials' mechanical properties, enhancing tensile strength, bending strength, abrasion resistance, and overall durability. It also improves the surface finish, reducing friction and making materials easier to handle, thus extending the product's lifespan.
Aerospace and Automotive Industry: Nickel and copper-nickel alloys are plated onto aircraft bodies, structural elements, and chassis parts to improve toughness and bending strength. Hard chrome plating is extensively used in engine parts, bearings, and gears to enhance wear resistance and impact durability.
Tool and Mold Manufacturing: Nickel and cobalt coatings are applied to tools and molds to increase tensile strength and wear resistance, enabling them to withstand high-stress conditions. Hard chrome is also popular for its wear resistance and ability to minimize material adhesion.
3D Printing and Plastic Products: Nickel plating is used on 3D-printed SLA resins and plastic products to enhance tensile and bending performance, bringing their mechanical properties closer to those of metals.
Improved Chemical Properties (Corrosion, Chemical, UV and Radiation Resistance)
Electroplating creates a protective barrier that enhances resistance to corrosion, chemicals, UV rays, and radiation, extending the lifespan of materials used in harsh environments.
Medical Industry: Gold and titanium coatings are used on medical devices like heart stents, joint prostheses, and dental implants due to their high biocompatibility and corrosion resistance in body fluids. Silver plating, with its antibacterial properties, is applied to catheters and other devices to reduce the risk of infection.
Marine Industry: Zinc plating protects large marine structures like ship decks, railings, and frames from saltwater and humidity. Electroless nickel plating is used on pipelines and valves to withstand harsh maritime conditions.
Chemical Industry: Titanium coating is favored for equipment in the chemical industry because of its resistance to strong acids and alkalis. It's used on chemical reactors, storage tanks, and industrial evaporators to ensure stable operation.
Aerospace Industry: Spacecraft and satellites are often plated with aluminum and gold to protect against intense ultraviolet rays and cosmic radiation. Nickel plating also offers additional protection against atmospheric corrosion.
The Limitations of Electroplating
Despite its many advantages, electroplating has several limitations:
Environmental Impact
Electroplating uses hazardous chemicals such as cyanide, heavy metals, and acids. Improper management can lead to environmental pollution. Disposing of hazardous waste and treating wastewater is costly and must comply with strict environmental regulations to prevent contamination. Additionally, electroplating is energy-intensive, especially in large-scale production, as it requires a continuous supply of direct current. This high energy consumption increases production costs and contributes to a larger carbon footprint.
Complex Process
The outcome of electroplating depends on precise control of multiple parameters, including current density, electrolyte temperature and concentration, and careful management of the pretreatment process. Different substrates have varying compatibility with electroplating solutions. Some metals may corrode electrochemically or react adversely in certain solutions, preventing even coating adhesion.
Time-Consuming Process
Electroplating can be a slow process, especially when applying high-quality or thick coatings. Increasing the power supply or electrolyte concentration to speed up the process often results in uneven coatings, compromising quality. This extended processing time can delay production schedules and reduce manufacturing efficiency.
Limited Coating Thickness
Electroplating is mainly suitable for thin coatings, usually ranging from a few micrometers to a few hundred micrometers. For applications requiring thicker, more durable coatings, methods like thermal spraying, cladding, or hot-dip galvanizing are more appropriate.
Surface-Only Benefits
The advantages of electroplating are limited to the surface layer. Once the coating is scratched or worn off, the underlying material is exposed, losing the performance benefits of the plating. This makes it less suitable for applications that require deep or structural protection.
Conclusion
Electroplating is a powerful technique that significantly enhances material properties and is widely used across various industries. However, achieving consistent results can be challenging. Partnering with a professional electroplating supplier is key.
At HL Parts, our team of experienced engineers, with over a decade of electroplating expertise, is the ideal partner for your projects. With advanced manufacturing capabilities, including CNC machining and sheet metal fabrication, along with in-house facilities and a robust network, we deliver high-precision metal parts while reducing lead times. Reach out to us today for expert electroplating solutions!